
quantusFLUNG
The First 100% Non-Invasive Fetal Lung Maturity Test in the Market
What is quantusFLUNG?
quantusFLUNG is an 100% non-invasive Fetal Lung Maturity test based on the analysis of an ultrasound image of the fetal lungs.
quantusFLUNG works as a web application that allows you to upload the ultrasound images for automatic analysis and review the reliable results within minutes.
quantusFLUNG offers you an unprecedented solution to predict the risk of Neonatal Respiratory Morbidity in a non-invasive, reliable and fast manner.
Comparision of quantusFLUNG and other commerical FLM tests
| Sensitivity | Specificity | PPV | NPV | |
| L/S Ratio A | 74.6% | 82.5% | 34.1% | 94.4% |
| PG A | 82.7% | 54.4% | 18.0% | 96.3% |
| Lamellar Body A | 84.2% | 74.4% | 27.9% | 97.6% |
| quantusFLUNG B | 71.0% | 94.7% | 67.9% | 95.4% |
| L/S: Lecithin / Sphingomyelin PG: Phosphatidol Glycerol |
A Average reported values (references 1-6) in clinical studies B Reported results in clinical study (reference 22) |
Why quantusFLUNG is different?
quantusFLUNG provides a NON-INVASIVE and EFFICIENT solution to determine the Fetal Lung Maturity.
Current FLM tests based on amniotic fluid analysis




quantusFLUNG





The web-based solution enables TIMESLESS and BORDERLESS user experience.
 Unrestricted and 24/7 access: As long as there is Internet, you can use quantusFLUNG and review the results ANYTIME, ANYWHERE.
Unrestricted and 24/7 access: As long as there is Internet, you can use quantusFLUNG and review the results ANYTIME, ANYWHERE.
 No installation required: quantusFLUNG is designed to give new users an easy start because neither downloading nor installation of any software is required.
No installation required: quantusFLUNG is designed to give new users an easy start because neither downloading nor installation of any software is required.
 Great compatibility: quantusFLUNG is compatible with major web browsers as well as most commonly-used Obstetrics and Gynecology Ultrasound Machines.
Great compatibility: quantusFLUNG is compatible with major web browsers as well as most commonly-used Obstetrics and Gynecology Ultrasound Machines.
How is quantusFLUNG useful?
Determining Fetal Lung Maturity is a Historical Need
Information about Fetal Lung Maturity* may be of help to assess the decision of deliverying when the balance between the risk of Neonatal Respiratory Morbidity** and the fetal or maternal risk to prolong pregnancy is not clear.
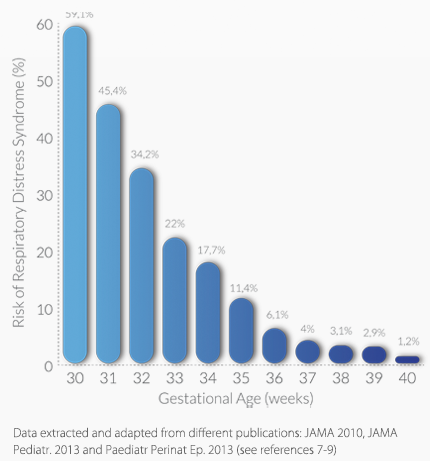
Maternal or fetal conditions such as moderate preeclampsia, diabetis, cholestasis or growth restriction may need to plan delivery before the spontaneous labor occurs. Although Neonatal Respiratory Morbidity is much more frequent in early preterm babies, it also occurs in late preterm babies (>34 weeks) and even in early term newborns.
For that reason, reference associations like ACOG recommend that obstetricians confirm fetal pulmonary maturity prior to elective delivery with less than 39 weeks gestation. That information, directly related with the risk of Neonatal Respiratory Morbidity, is advisable in order to plan place and time of delivery together with the neonatologists.
However, the main problem is that all the present FLM tests require an invasive amniocentesis procedure that not only causes discomfort to the patients but also poses potential complications. Therefore, despite the majority of the clinicians consider conducting FLM test important, the intrinsic complications of the current methods greatly compromise its massive use.
* The term "Fetal Lung Maturity" is universally used by the scientific and medical community to define the capacity of fetal lungs to achieve normal respiratory function if the fetus is born.
** Defined as either Respiratory Distress Syndrome or Transient Tachypnea of the newborn that requires his admission to a special unit and the use of medical respiratory support.
When to use quantusFLUNG?
From a Evidence-Based Medicine to a Personalized Medicine
quantusFLUNG can be particularly useful where elective delivery could be an acceptable option but the risk of neonatal respiratory morbidity should be known. In many clinical situations the decision of whether to deliver or wait is in a "grey zone", particularly in late preterm to early-term (34+0 to 38+6 weeks) pregnancies. Typical examples can be:
- Difficult-to-control hypertension or diabetes
- Moderate preeclampsia
- Maternal fluid retention with edema
- Very symptomatic cholestasis
- Previous history of unexplained fetal death or abruption
- Any situation where an elective cesarean section <39+0 weeks is considered
In these and other circumstances delivery may be a reasonable, but not an absolute, option to avoid danger to mother or fetus. Knowing the risk of neonatal respiratory morbidity in a non-invasive manner, can be a critical information in the decision-making process, either to confirm or otherwise delay delivery.
For instance, in a 36+0 week pregnancy, the baseline risk of morbidity and NICU admission for respiratory support is 6.1%. However, a risk adjusted by quantusFLUNG below the baseline risk might reduce the chances of morbidity to 5.2%, while if the risk adjusted by quantusFLUNG is above the baseline risk, the probability of respiratory morbidity migth be 33.7%. Thus, knowing FLM (without the need of an invasive technique) may have a clear impact in the clinical management of this case.
Why does quantusFLUNG work?
Changes occurring at the histological level of a tissue, including the proportion of collagen, fat or water, among others, affect ultrasound backscattering signals. This constitutes the basis for ultrasound image reconstruction. Computerized quantitative ultrasound analysis detects extremely subtle changes, unpercievable by the human eye, in order to accurately infer relevant information of tissue microstructure.
Fetal Lung Maturity constitutes an obvious candidate for the use of quantitative ultrasound solutions as fetal lung maturity results from the combination of the evolving changes in lung airways and alveoli during gestation, and the concentration of surfactant. Over the last 30 years, research has focused on the extraction of quantitative information about tissue characteristics from ultrasound images.
quantusFLUNG brings the opportunity to avoid the need of using an invasive technique to predict neonatal respiratory morbidity in the clinical practice. Transmural Biotech's quantusFLUNG software uses a combination of cutting-edge image analysis technologies that make individualized predictiveness of the risk of Neonatal Respiratory Morbidity.
quantusFLUNG reaches unprecedented levels of accuracy and reproducibility for a completely non-invasive ultrasound-based test.
References:
- A comparison of the accuracy of the TDx-FLM assay, Lecithin-Sphingomyelin Ration, and Phosphatidyglycelrol in the prediction of Neonatal Respiratory Distress Syndrome. E. Hagen, JC. Link and F. Arias. Obstet Gynecol (1993) 82, 1004-8.
- A Direct Comparison Between Lamellar Body Counts and Fluorescent Polarization Methods for Predicting Respiratory Distress Syndrome. S. Haymond, VI. Luzzi, CA. Parvin and AM. Gronowski. Am J Clin Pathol (2006) 126,894-899.
- Gestational age-specific predicted risk of neonatal respiratory distress syndrome using lamellar body count and surfactant-to-albumin ratio in amniotic fluid. R. Karcher, E. Sykes, D. Batton,Z. Uddin, G. Ross, E. Hockman and GH. Shade Jr. AJOG (2005) 193, 1680–4.
- Lamellar Body Counts Compared With Traditional Phospholipid Analysis as an Assay for Evaluating Fetal Lung Maturity. MG. Neerhof, EI. Haney,RK. Silver, ER. Ashwood, IS Lee and JJ. Piazze. Obstet Gynecol (2001) 97, 305–9.
- Multicenter Evaluation of TDx Test for Assessing Fetal Lung Maturity. JC. Russell, CM. Cooper, CH. Ketchum, JS. Torday, DK. Richardson, JA. Holt, LA. Kaplan, JR. Swanson and WM. Ivie. Clin Chem (1989) 35/6, 1005-1010.
- Neonatal morbidity after documented fetal lung maturity in late preterm and early term infants. BD. Kamath, MP. Marcotte and EA. DeFranco. AJOG (2011) 204, 518.e1-8.
- Quantitative Ultrasound Texture Analysis of Fetal Lungs to Predict Neonatal Respiratory Morbidity. Bonet-Carne E, Palacio M, Cobo T, Perez-Moreno A, Lopez M, Piraquive JP, Ramirez JC, Marques F, Gratacos E. Ultrasound Obstet Gynecol. 2014 Jun 11. doi: 10.1002/uog.13441.
- Adverse neonatal outcomes associated with early-term birth. S. Sengupta, V. Carrion, J. Shelton, R.J. Wynn, R.M. Ryan, K. Singhal and S. Lakshminrusimha. JAMA Pediatr. 2013 Nov 1;167(11):1053-9.
- Respiratory morbidity in late preterm births. Consortium on Safe Labor. JAMA. 2010 Jul 28;304(4):419-25
- Risk factors for acute respiratory morbidity in moderately preterm infants. M. Altman , M. Vanpée , S. Cnattingius and M. Norman. Paediatr Perinat Epidemiol. 2013 Mar;27(2):172-81.
- Changing patterns of fetal lung maturity testing. K.T. McGinnis, J.A. Brown and J.C. Morrison. Journal of Perinatology. 2008 Jan; 28(1):20-3.
- Clinical and laboratory trends in fetal lung maturity testing. D. G. Grenache , A.R. Wilson, G.A. Gross and A.M. Gronowski. Clin Chim Acta. 2010 Nov 11;411(21-22):1746-9.
- Effectiveness of antenatal corticosteroids in reducing respiratory disorders in late preterm infants: randomised clinical trial. A.M. Porto, I.C. Coutinho, J.B. Correia and M.M. Amorin. BMJ. 2011 Apr 12; 342:d1696.
- Monitoring structural changes in cells with high-frequency ultrasound signals statistics. A.S. Tunis, G.J. Czarnota, A. Giles, M.D. Sherar, J.W. Hunt, and M.C. Kolios. Ultrasound in Med and Biol. 2005 Aug; 31(8):1041-9.
- Performance of an automatic quantitative ultrasound analysis of the fetal lung to predict fetal lung maturity. M. Palacio; T. Cobo, M. Martínez-Terrón, G. Rattá, E. Bonet-Carne, I. Amat-Roldan and E. Gratacos. Am J Obstet gynecol. 2012 Dec; 207(6):504.e1-5.
- Practice Bulletin Clinical Management Guidelines for Obstetrician. American College of Obstetricians and Gynecologists (ACOG). September 2008, Number 97.
- Revisiting Amniocentesis for Fetal Lung Maturity After 36 Weeks’ Gestation. G. Luo, and E.R. Norwitz. Rev Obstet Gynecol. 2008 Spring; 1(2): 61-68.
- Quantitative ultrasonography. M.F. Insana, B.S. Garra, S.J. Rosenthal and T.J. Hall. Med Prog Technol. 1989; 15(3-4):141–53.
- Theoretical framework for spectrum analysis in ultrasonic tissue characterization. F.L. Lizzi, M. Greenbaum, E.J. Feleppa, M. Elbaum and D.J. Coleman. J Acoust Soc Am. 1983; 73(4):1366-1373.
- The ultrasonic changes in the maturing placenta and their relation to fetal pulmonic maturity. P.A. Grannum, R.L. Berkowitz, and J.C. Hobbins. Am J Obstet Gynecol. 1979 Apr 15;133(8):915-22.
- An investigation of backscatter power spectra from cells, cell pellets and microspheres. M.C. Kolios MC, L. Taggart, R.E. Baddour, F.S. Foster, J.W. Hunt, G.J. Czarnota and M.D, Sherar. 2003 IEEE Symposium on Ultrasonics;1:752-57.
- Prediction of Neonatal Respiratory Morbidity by Quantitative Ultrasound Lung Texture Analysis: A Multicenter Study, American Journal of Obstetrics and Gynecology (2017), doi: 10.1016/j.ajog.2017.03.016.
quantusFLUNG:
A Revolution in Fetal Lung Maturity Tests

Non Invasive

Reliable

Fast



